Carbonic anhydrase inhibitors. Interaction of 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide with 12 mammalian isoforms: kinetic and X-ray crystallographic studies.
Guzel, O., Temperini, C., Innocenti, A., Scozzafava, A., Salman, A., Supuran, C.T.(2008) Bioorg Med Chem Lett 18: 152-158
- PubMed: 18024029
- DOI: https://doi.org/10.1016/j.bmcl.2007.10.110
- Primary Citation of Related Structures:
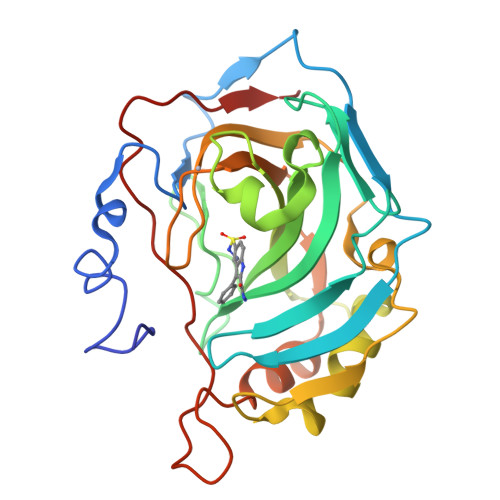
3B4F - PubMed Abstract:
2-(Hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide was tested for its interaction with 12 carbonic anhydrase (CA, EC 4.2.1.1) isoforms in the search of compounds with good inhibitory activity against isozymes with medicinal chemistry applications, such as CA I, II, VA, VB, VII, IX, and XII among others. This sulfonamide is a potent inhibitor of CA I and II (K(I)s of 7.2-7.5 nM), a medium potency inhibitor of CA VII, IX, XII, and XIV, and a weak inhibitor against the other ubiquitous isoforms, making it thus a very interesting clinical candidate for situations in which a strong inhibition of CA I and II is needed. The crystal structure of the hCA II adduct of this sulfonamide revealed many favorable interactions between the inhibitor and the enzyme which explain its strong low nanomolar affinity for this isoform but may also be exploited for the design of effective inhibitors incorporating bicyclic moieties.
Organizational Affiliation:
Istanbul University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, 34116 Beyazit, Istanbul, Turkey.